A little background. REACH = Registration, Evaluation, Authorisation and Restriction of Chemicals and is a European Union (EU) regulation that went into effect June 1, 2007. Officially known as Regulation (EC) No 1907/2006, it regulates the production and use of specific chemical substances in products imported into the EU that may negatively impact human health and the environment. This regulation also created the European Chemical Agency (ECHA) that administrates REACH.
REACH consists of three main substance lists of that are of concern to product manufacturers and importers:
1. Substances of Very High Concern (SVHC) Candidate List known as Annex I of Regulation (EC) No. 1907/2006
2. REACH Authorisation List known as Annex XIV
3. Restricted Substances List known as Annex XVII
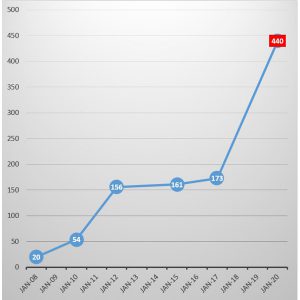
The SVHC Candidate List, which currently contains 173 substances at the time of this writing, has a reporting obligation. If you use any substance above a specific threshold in your products you must report the presence of these substances to the end user or to your customer. A recent update was the addition of Bisphenol A (BPA) to the SVHC Candidate List in December 2016. The effects were immediately felt in the consumer market because of the popular use of BPA in plastics, particularly in water drinking bottles.
After a two-step regulatory process, SVHCs may be included in the Authorisation List, which currently contains 31 substances at the time of this writing, and become subject to authorisation. These substances cannot be placed on the market or used after a given date, unless an authorisation is granted for their specific use, or the use is exempted from authorization. ECHA is continually reviewing new chemicals for their potential inclusion in the SVHC Candidate list and further inclusion in the REACH Authorisation List. The list also has variables on the dates of when these substances will be prohibited in products and provide for very limited use exemption/ authorisations. The list is continually reviewed for expiration or acceptance. This contributes to making REACH compliance a moving target.
the REACH Authorisation List. The list also has variables on the dates of when these substances will be prohibited in products and provide for very limited use exemption/ authorisations. The list is continually reviewed for expiration or acceptance. This contributes to making REACH compliance a moving target.
The REACH Authorisation list contains 12 substances that have a “sunset” date in 2017. “Sunset” date refers to the deadline when these substances will be prohibited in products imported or manufactured in the EU. There are additional substances with sunset dates as far out at 2019.
Furthermore, the Restricted Substances List are substances for which production, placing on the market or use, is limited or banned in the EU. This list currently contains 63 substances either on their own, in a mixture or in an article.
There are currently over 143,000 chemical substances and compounds being reviewed for registration. REACH is concerned not only with chemicals in the final product, but also regulates chemicals used in the manufacturing process. Companies that manufacture products in the EU or for export into the EU need to have a full and complete knowledge of the chemicals used in the manufacturing and final products they produce.
One of the key methods to staying ahead and on-target with the regulation compliance curve is a Full Material Disclosure (FMD) process tracking through the entire supply chain. This enables the manufacturer to already have the data to respond to changes and additions in the regulation lists. We have a team of supply chain professionals that work to implement and administrate FMD processes for clients. We collect laboratory reports on components, sub-assemblies and final products and input the data into a variety of customer owned Enterprise Database Tools. This gives the customer the objective 3rd party data to make data-backed DOC’s.
Watch our blog for a follow up post where we will review the SVHC list in more detail. For more information please visit the official ECHA REACH page.